14
2025
-
05
Preparation and spill handling of isobutyryl chloride
Source:
https://www.chemicalbook.com/NewsInfo_53082.htm
Author:
ChemicalBook
Isobutyryl chloride, also known as 2-methylpropionyl chloride, isobutyric acid chloride, is a colorless liquid with a pungent odor, soluble in ether, and decomposes upon contact with water.
Introduction
Isobutyryl chloride, also known as 2-methylpropanoyl chloride or isobutyric acid chloride, is a colorless liquid with an irritating odor. It is soluble in ether and decomposes upon contact with water. Isobutyryl chloride is an important intermediate in organic synthesis, as well as in the fields of pesticides and pharmaceuticals. Under the action of anhydrous AlCl3, isobutyryl chloride reacts with benzene to synthesize phenyl isopropyl ketone, which is an important intermediate for the new generation of photocurable coating photoinitiator 2-hydroxy-2-methyl-1-phenylpropanone. Generally, phosphorus trichloride is used as a chlorinating agent to synthesize isobutyryl chloride. Its byproduct is phosphorous acid, which easily transforms into a yellow solid phosphide at higher temperatures, which is not conducive to post-processing of production. Therefore, it is more important to study its simple synthesis method [1].
Preparation
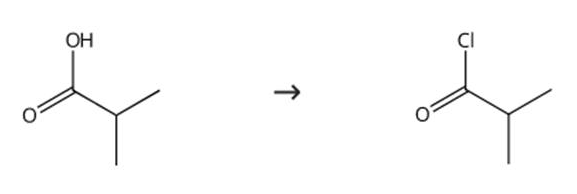
Figure 1 Synthesis route of isobutyryl chloride
Oxalyl chloride (10 g, 0.11 mol) was added dropwise to a solution in 20 mL DCM at 0 °C, followed by the addition of two drops of DMF. The mixture was stirred overnight at room temperature, then the mixture was distilled and the fraction was collected in the range of 66-72 °C to obtain the compound isobutyryl chloride with a yield of (6 g, 50%). The synthesis route is shown in Figure 1 [2].
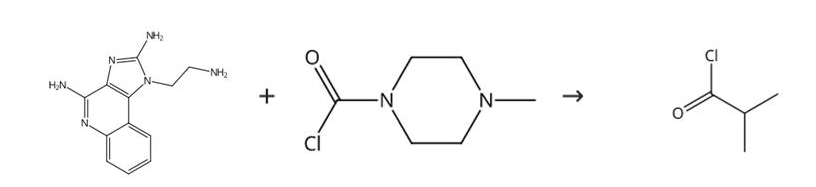
Figure 2 Synthesis route of isobutyryl chloride
Acid chloride, sulfonyl chloride, isocyanate or carbonyl chloride (0.105 mmol, 1.05 eq) was added to a test tube containing N,N-diisopropylethylamine (35.3 μL, 0.203 mmol) and 1-(2-aminoethyl)-1H-imidazo[4,5-c]quinoline-2,4-diamine (24.4 mg, 0.100 mmol) in DMA (1 mL). The tube was then capped and vortexed overnight at room temperature. Ammonium hydroxide (250 μL) was added to each tube and the contents were vortexed overnight at room temperature. Two drops of water were added to the test tube, and the solvent was removed by vacuum centrifugation. The compound isobutyryl chloride was then purified by reverse-phase preparative HPLC. The synthesis route is shown in Figure 2.
Leakage Handling
Once isobutyryl chloride is leaked, isolate the leaked contaminated area, set up warning signs around it, and cut off the fire source. Emergency responders should wear gas masks and general fire protective clothing. Collect the isobutyryl chloride with a clean shovel into a dry, clean, covered container and transport it to a waste disposal site.
References
[1] Wang Kehai, Research and development and pilot test of isobutyryl chloride. Gansu Province, Gansu Provincial Chemical Research Institute, 2005-01-01.
[2] Yanvarev, D. V.; Korovina, A. N.; Usanov, N. N.; Kochetkov, S. N. Non-hydrolysable analogues of inorganic pyrophosphate as inhibitors of hepatitis C virus RNA-dependent RNA-polymerase. Russian Journal of Bioorganic Chemistry (2012), 38(2), 224-229.
Previous page
Next page
Previous page:
Next page:
Related News