14
2025
-
05
Physicochemical properties and pharmaceutical applications of n-butyryl chloride
Source:
https://www.chemicalbook.com/NewsInfo_62594.htm
Author:
Circuit Diagram
Butyryl chloride, with the English name Butyryl chloride, is a transparent yellowish liquid at room temperature and pressure, has strong hygroscopicity and is relatively sensitive to water, and is soluble in common organic solvents including ethyl acetate and dichloromethane. Butyryl chloride belongs to the alkyl acyl chloride compounds, possesses extremely high chemical reactivity, and can undergo acylation reactions with common alcohols or amine compounds, widely applied in the structural modification and synthesis of pesticide molecules.
Butyryl chloride, a clear, slightly yellowish liquid at room temperature and pressure, is highly hygroscopic and sensitive to water. It is soluble in common organic solvents, including ethyl acetate and dichloromethane. Butyryl chloride, an alkyl acyl chloride, is highly reactive and undergoes acylation reactions with common alcohols and amines. It is widely used in the structural modification and synthesis of pesticide molecules.
Physicochemical Properties
The chemical reactivity of butyryl chloride is mainly concentrated in its acyl chloride unit. Under alkaline conditions, it can undergo condensation reactions with alcohols or amines to yield the corresponding esters or amides. Furthermore, catalyzed by Lewis acids such as aluminum trichloride, butyryl chloride can undergo Friedel-Crafts acylation reactions with aromatic compounds to produce phenylbutanones. This reaction is an important organic synthesis method used to construct acylated structures on aromatic rings.
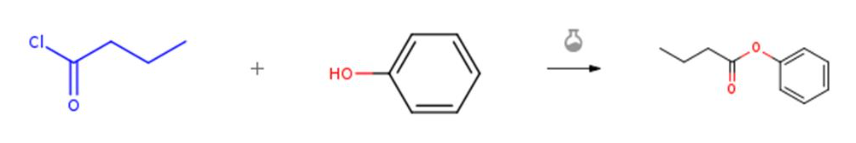
Figure 1 Condensation reaction of butyryl chloride and phenolic compounds
In a dry reaction flask, phenol (0.28 mmol) and butyryl chloride (0.84 mmol, 3 equivalents) were dissolved in a mixture of 1% triflic acid and acetonitrile (1 ml) at room temperature. The resulting reaction mixture was stirred vigorously at this temperature for approximately 1 hour. After the reaction, it was poured into cold water and ethyl acetate, then washed with 1 M HCl, saturated NaHCO 3 and saturated NaCl. The organic layer was separated, dried over anhydrous MgSO4, filtered, and concentrated under vacuum to obtain the target product molecule. [1]
Pharmaceutical Applications
The high reactivity and diverse reaction pathways of butyryl chloride make it an important tool compound in organic synthesis, playing an important role in the production of pesticides and pharmaceuticals. It serves as a building block for the synthesis and development of new compounds. Butyryl chloride can be used as an organic synthesis intermediate and a basic raw material in pharmaceutical chemistry. In pesticide production, it is used in the preparation of herbicides such as bensulfuron-methyl, tembotrione, and thifensulfuron-methyl. In drug molecule synthesis, it is used in the synthesis of drugs such as indomethacin and meperidine.
References
[1] Murashige, Ryo; Tetrahedron, 2011, 67, 641-649.
Related News