Acid halides are derivatives obtained by replacing the hydroxyl group in carboxylic acids with halogens (fluorine, chlorine, bromine, iodine). Acid halides are highly reactive compounds that do not exist in nature and can only be produced through chemical synthesis.
Acid chlorides are the most important and widely used acid halides, widely used in the construction of amide bonds, ester bonds, etc. This article will discuss them.
Acid chlorides are classified into higher acid chlorides and lower acid chlorides according to the number of carbons. Lower acid chlorides are pungent-smelling liquids, some of which are extremely foul-smelling (such as pivaloyl chloride); higher acid chlorides are solids, and the odor is usually much weaker.
Due to the absence of association in the molecule, the boiling point of acid chlorides is lower than that of the corresponding carboxylic acids. Acid chlorides are insoluble in water, and lower ones decompose upon contact with water. Due to the strong electronegativity of chlorine, the main manifestation in acid chlorides is a strong electron-withdrawing inductive effect, while the conjugation effect with the carbonyl group is very weak. Therefore, the C-Cl bond in acid chlorides is not shorter than the C-Cl bond in alkyl chlorides.
Due to the high reactivity of acid chlorides, in organic synthesis, inactive acids are often activated into acid chlorides, and then reacted with the corresponding alcohols or amines to obtain the corresponding esters or amides. This is also the most common and common use of acid chlorides. Compared with converting carboxylic acids into activated esters, this process usually maintains a good cost. In addition, acid chlorides usually have the following uses;
① Reaction with organometallic reagents, such as the reaction of acid chlorides with one molecule of Grignard reagent to generate ketones, and then the reaction with the second molecule of Grignard reagent can further convert the ketone into a tertiary alcohol;
② Under the conditions of Lewis acid AlCl3, etc., acid chlorides can undergo electrophilic aromatic substitution reactions (Friedel-Crafts reactions) with aromatic compounds;
③ Acid chlorides can also be reduced to aldehydes under certain conditions (Rosenmund reduction);
④ Acid chlorides are also used to react with sodium peroxide to generate peroxyacyl compounds. Peroxyacyl compounds contain a peroxy bond -O-O-, and under heating or light irradiation, unstable peroxyacyls decompose, releasing CO2 and generating free radicals, which are commonly used initiators for free radical reactions.
There are many methods for preparing acid chlorides. Commonly used chlorinating agents include oxalyl chloride, thionyl chloride, phosphorus oxychloride, phosphorus pentachloride, triphosgene, and chlorine, but the first three are widely used. Their shadows can be seen in the synthesis of many bulk commodity intermediates!
Oxalyl chloride (boiling point: 62-65 °C), thionyl chloride (boiling point: 79 °C), and phosphorus oxychloride (boiling point: 107 °C) are generally interchangeable under normal conditions. However, there is a priority. If the boiling point of the product is relatively low, distillation can be used for purification, and phosphorus oxychloride can be prioritized; at the same time, considering the by-products of the three reactions, if the carboxyl raw material has acid-labile groups such as Boc, it is usually better to use an equivalent of oxalyl chloride.
Commonly used solvents for synthesizing acid chlorides include dichloromethane, chloroform, toluene, dioxane, etc. A very small amount of DMF is usually added as a catalyst to accelerate the reaction. The principle is to use the nucleophilicity of the lone pair of electrons on the oxygen of DMF and the electrophilicity of the chlorinating agent as the driving force to carry out a series of reactions to generate more active Vilsmeier reagent, and then quickly react with the acid to generate acid chloride (this process usually reacts very violently, and a large amount of bubbles are generated quickly after adding DMF).
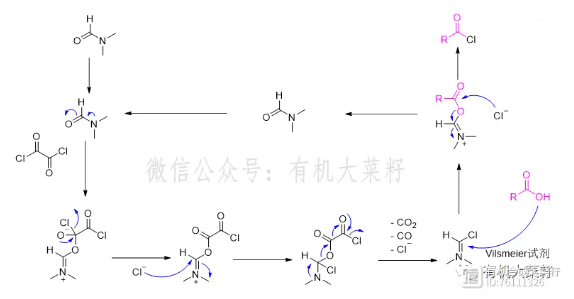
Figure, DMF-catalyzed acid chloride synthesis mechanism (taking oxalyl chloride as an example)
The general steps for synthesizing acid chlorides are: first dissolve the acid in a solvent, stir and cool to below 0 °C, slowly add the chlorinating agent, add it completely, and raise the temperature to room temperature for reaction. Most substrates can react completely in 2-3 hours; for sterically hindered substrates, the temperature can be raised to reflux or the time can be extended.
For systems with insoluble substances after the reaction, TLC must be carefully checked. Generally, acids with high polarity will show a difficult-to-dissolve state, but the polarity will become very small after being made into acid chlorides, and they will basically dissolve. When monitoring TLC plates, because the activity of acid chlorides is too high, direct TLC is meaningless.
The general procedure is to take a little and add it to methanol to quench it to generate the corresponding ester, and then confirm the extent of the reaction by TLC.

Figure, TLC of acid chloride esterification
In esterification and amidation reactions, the prepared acid chlorides are generally used immediately and rarely purified. After the reaction solution TLC or other methods such as nuclear magnetic resonance are used to identify the disappearance of the raw materials, the solvent and acid gas are usually removed by rotary evaporation, and the resulting residue is usually washed with toluene once or twice. Finally, the residue is cooled under nitrogen, and fresh dry solvent is added to completely dissolve it, and then it is added dropwise to react with the corresponding substance.
During esterification and amidation, some people like to be lazy and don't want to use rotary evaporation. There is also a solution, which is to add an excess of acid-binding agent to the system to ensure that the system is alkaline!
When using phosphorus pentachloride, triphosgene, chlorine, etc. as chlorinating agents for chlorination, specific problems should be analyzed specifically, and attention should be paid to what the reaction by-products are and how toxic they are.
For example, when using triphosgene for chlorination, especially when scaling up, a tail gas treatment device should generally be added. After the reaction is completed, rotary evaporation should be carried out in a fume hood, or the next step should be carried out directly in an alkaline environment. Therefore, when it is involved in use, those who have the conditions should wear a phosgene monitoring test paper on their chest, and should not be ignorant and fearless.
Acid chloride synthesis example:
(1) Thionyl chloride method (reference: Org. Lett. 2009, 11, 12, 2659–2662)
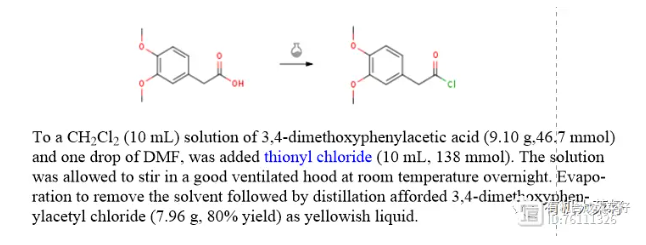
(2) Oxalyl chloride method (Reference: Arkivoc, 2018, 5, 334 - 347)
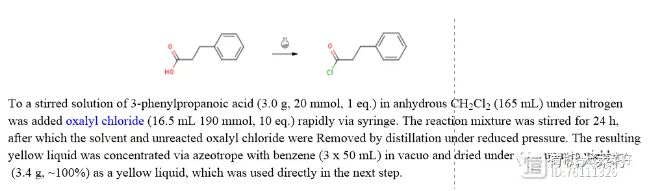
(3) Triphosgene method (with hydroxyl groups, Reference: Bioorg. Med. Chem. Lett. 74 (2022) 128920)
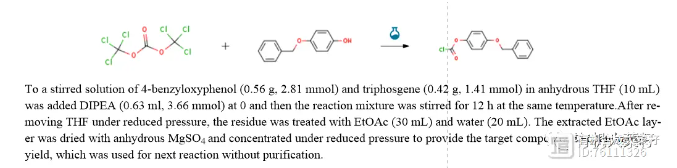
Other questions:
Can water be used as a solvent when the acid chloride undergoes the next reaction?
There is no uniform answer to this question; it needs to be analyzed on a case-by-case basis. Generally, it is not recommended for esterification, Friedel-Crafts reactions, etc., but for amidation reactions, water can often be used as a solvent, and an inorganic base can be used as an acid scavenger, for example:
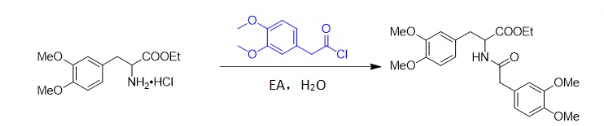
This is because, compared to water, amines have higher nucleophilicity and have priority in choosing partners. As long as it can be stirred evenly, water is always inferior!