14
2025
-
05
Acid chloride
Source:
https://m.chemicalbook.com/NewsInfo_1371.htm
Author:
ChemicalBook
Acid chlorides are important carboxylic acid derivatives with significant applications in organic synthesis and pharmaceutical synthesis. They can undergo various reactions, including hydrolysis, alcoholysis, ammonolysis (aminolysis), reactions with organometallic reagents, reduction reactions, and α-hydrogen halogenation.
Preparation Method
1. Phosphorus trichloride method (1) Propionic acid reacts with phosphorus trichloride to generate propionyl chloride. The reaction formula is as follows:
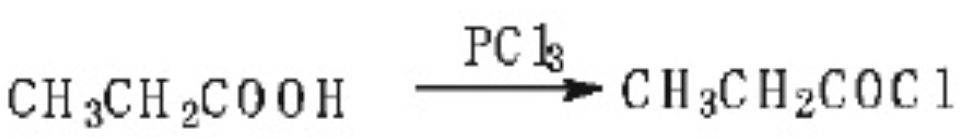
Propionamide is mainly used to synthesize the anti-epileptic drug methsuximide, cholagogue, and the anti-adrenergic drug methoxamine hydrochloride. In organic synthesis, it is used as a propionylation reagent. (2) Lauric acid reacts with phosphorus trichloride to generate lauroyl chloride, the reaction is as follows:

This product is used to synthesize lauroyl peroxide and sodium lauroyl polyamino acid. When using phosphorus trichloride to prepare acyl chloride, it is suitable for preparing low-boiling point acyl chlorides, because the phosphoric acid generated in the reaction is not easily volatile, and the acyl chloride can be easily distilled out.
2. Phosphorus pentachloride method
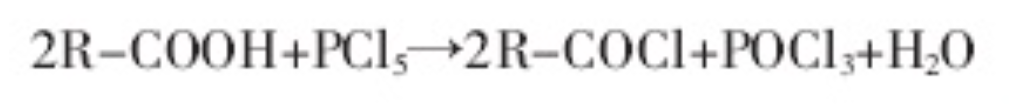
Because the boiling point of phosphorus oxychloride is relatively low, phosphorus pentachloride is generally suitable for preparing high-boiling point acyl chlorides, so that phosphorus oxychloride can be distilled out and separated. The phosphorus pentachloride method is less used, and there are few literature reports.
3. Thionyl chloride method (1) Conventional synthesis method Thionyl chloride is an acylating reagent that has been used for a long time. Because it has the advantages of mild reaction conditions, simple post-processing, and high reaction yield, it is widely used in scientific research and industry, and this method is still used at home and abroad.
a. Synthesis of isobutyryl chloride
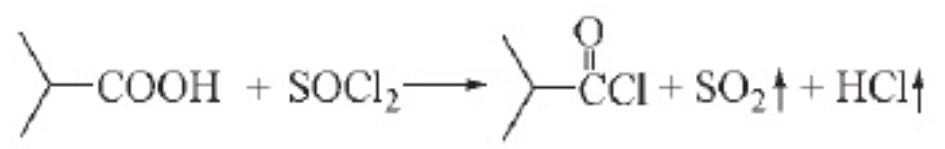
n(thionyl chloride):n(isobutyric acid)=1.2:1, reaction temperature 80℃, reaction time 45min, under this condition, the yield can reach 78%. Studies have found that the appropriate addition of phosphorus trichloride can further improve the yield of isobutyryl chloride to 84.5%.
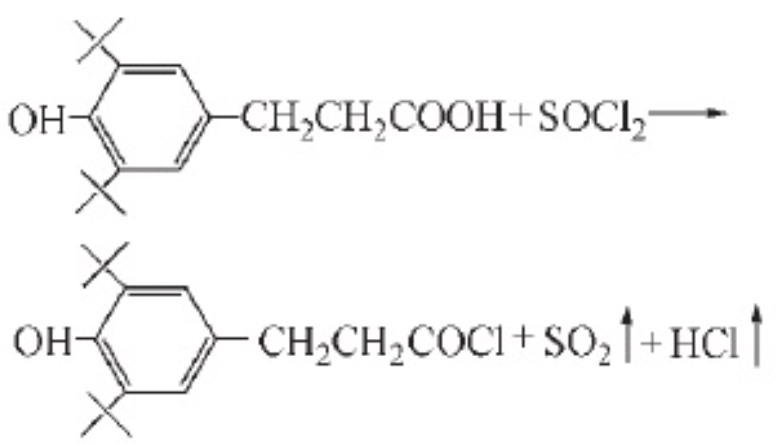
Using chloroform as a solvent, β-(3,5-di-tert-butyl-4-hydroxyphenyl)-propionic acid 0.02mol, thionyl chloride 0.054mol, reaction temperature 50℃, reaction time 5h, under this condition, the product yield is 98.9%. However, the authors of the literature found that only high-purity thionyl chloride can obtain high-purity target products, so reagent-grade thionyl chloride needs to be redistilled or double-distilled before being used for synthesis.
(2) Phase transfer catalysis method Phase transfer catalysis technology has only been newly developed in recent years. It has the advantages of simple operation, low reaction temperature, good selectivity, few side reactions, cheap catalyst price, recyclability, easy separation, and broad application prospects. It will become a research and development hotspot. Thionyl chloride method has many advantages, especially when combined with phase transfer catalysis, ultrasonic radiation and other methods, the advantages are even more obvious, but there are also many defects. First, this process is accompanied by the generation of toxic gas sulfur dioxide, which increases the cost of subsequent processing; at the same time, thionyl chloride itself is toxic and has a strong irritating smell, which is not conducive to green environmental protection; secondly, the production process is generally an excess of thionyl chloride, so additional distillation equipment needs to be used to recover it, which increases the cost, and the process may be accompanied by dehydration of thionyl chloride, resulting in the product turning black.
4. Phosgene method Phosgene is a very good acylating reagent. The product content and yield of acyl chloride prepared by phosgene are high. However, phosgene is a highly toxic gas, and it has great risks during use, transportation, and storage. Therefore, the phosgene route is avoided in both laboratory and industrial production.
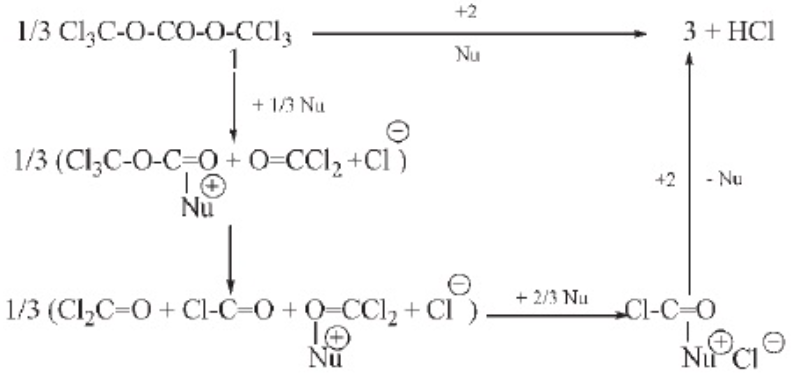
5. Solid phosgene method Solid phosgene is a stable white crystal with a melting point of 80℃ and a boiling point of 206℃. Even when boiling, only a small amount is decomposed to release phosgene, so it is extremely safe during transportation, storage, and use, and is treated as a general toxic substance. Solid phosgene can be used to prepare carbonates, haloalkanes, acyl chlorides, anhydrides, symmetrical and asymmetrical ureas, and five- or six-membered heterocycles, and can be used as an ideal substitute for phosgene and diphosgene. Its reaction mechanism is similar to that of phosgene and diphosgene. One molecule of solid phosgene can release three molecules of phosgene under the action of a nucleophile. The reaction mechanism is as follows.
6. Carbon tetrachloride method Using organic carboxylic acids as raw materials and carbon tetrachloride as an acylating agent can generate many important high-value-added organic intermediates, with good economic benefits and environmental friendliness.
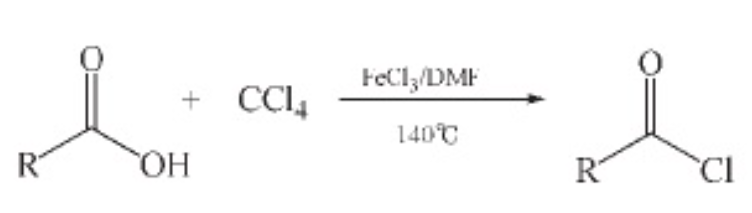
Carbon tetrachloride as an acylating agent is applicable to a large number of carboxylic acids, and the yield is relatively high. Compared with the solid phosgene method, the conditions may not be so mild, but compared with thionyl chloride, for some reactions, in the presence of ferric chloride, it can completely replace thionyl chloride.
Application
1. Application of acyl chlorides in medicine Acyl chloride compounds occupy an important position in new medicines. Using the active groups and catalytic effects in acyl chloride compounds, a series of pharmaceutical chemical products and intermediates can be synthesized, and some preparation methods of pharmaceutical derivatives can be provided. For example, 2,6-dichloro-5-fluoronicotinoyl chloride obtained by β-ketoesterification of 2,6-dichloro-5-fluoronicotinoyl ethyl acetate can be used as a key intermediate for naphthyridine ring fluoroquinolone drugs such as enoxacin and gatifloxacin; linoleic acid and vitamin E synthesized linoleoyl chloride is an important intermediate for the synthesis of vitamin E linoleate, vitamin E linoleate can be used as a tumor inhibitor, and can also be used to prepare high-end cosmetics, which have nutritional, moisturizing and anti-aging effects on skin and hair; N-4-hydroxyphenethyl-4-chlorobenzamide synthesized from p-chlorobenzoyl chloride and p-hydroxyphenethylamine is an important intermediate for the synthesis of benzafibrate, which can be used to regulate lipid metabolism disorders and is clinically used to treat type IIb, III and IV hyperlipidemia. Using acyl chlorides as raw materials, various acyl thioureas can be easily synthesized by phase transfer catalysis, thiourea method, and traditional synthesis methods. By using different acyl chlorides or reacting with different amines, various acyl thiourea pharmaceutical intermediates or products with anticancer activity, antibacterial activity, antiviral activity and other biological activities can be synthesized.
2. Applications of acyl chlorides in pesticides: High efficiency, low toxicity, low cost, and environmental friendliness are the basic requirements for the creation of new pesticides. Using acyl chlorides as intermediates, many novel pesticides with high biological activity can be synthesized through alcoholysis or aminolysis, as well as modification of substituents and splicing of active structures (such as the introduction of groups such as highly bioactive pyridine heterocycles, benzheterocycles, oxadiazines, and fluorine atoms). Acetyl oxadiazine compounds synthesized by acylating oxadiazine compounds using chloroacetyl chloride as an acylating agent are important intermediates in the synthesis of neonicotinoid pesticides. Pesticides with this structure have lower application dosages and broader insecticidal spectra compared to traditional pesticides. Acylthioureas synthesized using acyl chlorides as raw materials can also synthesize various novel pesticides or pesticide intermediates by modifying the substituents at the ends of the thiourea bridge.
3. Applications of acyl chlorides in resource and environment: Acylthiourea derivatives synthesized using acyl chlorides as intermediates, due to their rich sulfur and oxygen atoms, are strong electron donors. When combined with suitable metal ions, they can undergo complexation reactions with metal ions through the sulfur atoms on the ligands and the oxygen atoms on the acyl groups, making them good metal chelating agents.
【Main References】
[1]Yu Mingqiang. Synthesis and performance research of acyl chlorides [D]. Central South University, 2012.
[2]Yu Mingqiang, Liu Guangyi, Yin Xingrong, Cheng Qin, Di Ning. New progress in synthesis methods and applications of acyl chlorides [J]. Fine Chemicals Intermediates, 2011, 41(04): 1-7.
[3]Xin Jianfeng, Ma Jihai, Zhang Shufen, Chen Shaorui, Li Haiyu. Review of acyl chloride preparation methods [J]. Hebei Chemical Industry, 2006(11): 16-18+20.
Previous page
Related News