14
2025
-
05
Acylation reaction under the action of acyl chloride
Source:
https://www.chemicalbook.com/NewsInfo_64304.htm
Author:
ChemicalBook
Acyl chloride, whose English name is Acyl chloride, is a highly chemically reactive acylation reagent. It is a general term for a class of organic compounds, possessing strong hygroscopicity and deliquescence, and capable of undergoing vigorous hydrolysis reactions with water.
Acyl chlorides, also known as acyl chloride, are highly reactive acylating agents. They are a class of organic compounds characterized by strong hygroscopicity and deliquescence, undergoing vigorous hydrolysis with water. Acyl chlorides are typically prepared by the chlorination of carboxylic acids with thionyl chloride. They react with common nucleophiles such as alcohols and amines in acylation reactions, finding extensive applications in fundamental chemical research and pharmaceutical production.
Chemical Properties
Acyl chlorides are compounds containing the -COCl functional group, belonging to the acyl halide class. They are carboxylic acid derivatives formed by replacing the hydroxyl group in carboxylic acids with chlorine. The simplest acyl chloride is formyl chloride, but it is highly unstable and cannot be obtained from formic acid and chlorinating agents like other acyl chlorides. Common acyl chlorides include acetyl chloride, benzoyl chloride, oxalyl chloride, chloroacetyl chloride, trichloroacetyl chloride, and phosgene. These substances exhibit extremely high electrophilicity, readily undergoing condensation reactions with water and alcohols. These reactions are widely used in the acylation of alcohols and amines. It's noteworthy that these reactions often require the addition of a base as an acid scavenger to facilitate the reaction.
Acylation Reaction
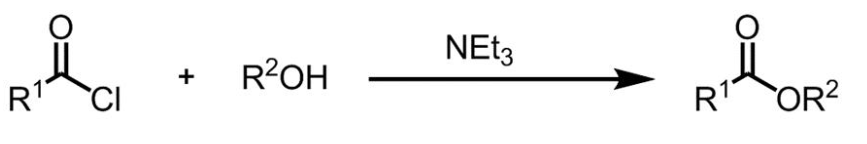
Figure 1 Acylation reaction of acyl chlorides with alcohols
In a dry reaction flask, acyl chloride (25 mmol) and triethylamine (2.53 g, 3.5 mL, 25 mmol) were added to a solution of alcohol or amine (25 mmol) in dry acetonitrile (30 mL). The resulting reaction mixture was stirred vigorously at room temperature for 4 hours. The reaction progress was monitored by TLC. After completion, water and ethyl acetate were added to the mixture for extraction. The organic layer was separated, dried over anhydrous MgSO4, filtered, and the filtrate was evaporated under vacuum. The residue was purified by silica gel column chromatography to obtain the target product molecule. [1]
Chemical Applications
In organic synthesis, acyl chlorides primarily serve as acylating agents, reacting with nucleophiles such as alcohols and amines in acylation reactions. This common organic synthesis reaction is used to synthesize esters and amides. Acyl chlorides, as common reagents in acylation reactions, introduce acyl functional groups into synthesized compounds.
References
[1] Xing Qiyi, Pei Weiwei, Xu Ruiqiu. Basic Organic Chemistry. Lower Volume [M]. Higher Education Press, 2005.
Previous page
Next page
Previous page:
Next page:
Related News